Ames Plate Incorporation Assay Kits
Salmonella Mutagenicity Complete Test Kit
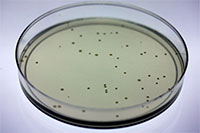
Provides all essential items for conducting the bacterial mutagenesis assay described by Maron and Ames1
The Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 are included in the kit. The lyophilized strains are fully characterized for phenotypic characteristics and respond to diagnostic mutagens as required by OECD Guideline 4713.
Kit contents:
- STDisc™ TA98, TA100, TA1535, TA1537 (10 discs/vial each)
- Lyophilized S9 (2 vials / 2.1 ml each)
- NADPH Regensys™ (40 ml Regensys A and 123 mg Regensys B)
- CONTROLCHEM™ pre-weighted positive control chemicals: ICR191, Daunomycin, Sodium azide, 2-Aminoanthracene
- Nutrient broth, Histidine/Biotin Top Agar, ST QUAD PC™ plates
- Minimal Glucose Agar (MGA) plates (160 dishes), Nutrient Agar plates (20 dishes)
- Instruction manual
| Catalog no. | Description | Storage | Size |
|---|---|---|---|
| 31-100.2 | Salmonella Mutagenicity Complete Test Kit | - | 1 sample kit |
This kit can be upgraded by substitution or addition of our E. coli or Salmonella typhimurium strains to comply with the OECD Guideline 4713. For high-throughput-screening please ask for bulk ware.
E. coli Mutagenicity Complete Test Kit
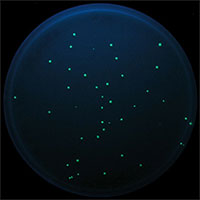
Provides all essential items for conducting the bacterial mutagenesis assay described by Green and Muriel2
Escherichia coli strains ECDisc™ WP2 trp and WP2 trp, uvrA are included in the kit. The lyophilized strains are fully characterized for phenotypic characteristics and respond to diagnostic mutagens as required by OECD Guideline 4713.
Kit contents:
- ECDisc™ WP2 trp and WP2 trp, uvrA (10 discs/vial each)
- Lyophilized S9 (2 vials / 2.1 ml each)
- NADPH Regensys™ (15 ml Regensys A and 46 mg Regensys B)
- CONTROLCHEM™pre-weighted positive control chemicals: Methylmethanesulfonate, 2-Aminoanthracene
- Nutrient broth, L-Tryptophan Top Agar, EC TRI PC™ plates
- Minimal Glucose Agar (MGA) plates (80 dishes), Nutrient Agar plates (20 dishes)
- Instruction manual
| Catalog no. | Description | Storage | Size |
|---|---|---|---|
| 31-101 | E. coli Mutagenicity Complete Test Kit | - | 1 sample kit |
µAmes 471 Bacterial Mutation Test Kit

24-well plate format, miniaturized Ames bacterial mutation test
The µAmes test allows Ames testing compliant with OECD guideline 4713 and is suitable to evaluate mutagenicity of pharmaceutical impurities when only limited amounts are available (see Guideline:ICH M74).
Advantages of the µAmes method:
- 95% less test article is required
- One technician may perform the test in a single day with results 3 days later
- Each bacterial strain is purified, fully characterized, lyophilized and provided with a quality control and formulation statement
- Phenotype-testing components confirm the characteristics of the cultures generated in your lab
Kit contents:
- Lyophilized strains Salmonella TA98, TA100, TA1535, TA1537 and E. coli WP2 trp, uvrA (2 discs/vial each)
- 10% S9 Mix, lyophilized ( MUTAZYME™ ) incl. sterile/deionized water for reconstitution
- 24-well plates with Minimal 0.4% Glucose Agar (22 dishes), Nutrient Agar plates (20 dishes),
- CONTROLCHEM™ pre-weighted positive control chemicals: Sodium azide, 9-Aminoacridine-HCl, 2-Nitrofluorene, 4-Nitroquinoline-N-oxide, 2-Aminoanthracene, Benzo(a)pyrene
- Nutrient Broth, Phosphate buffer, Top Agar His/Bio/Tryp
- Phenotype confirmation plates and 4 types of antibiotic discs
- Instruction manual
| Catalog no. | Description | Storage | Size |
|---|---|---|---|
| 31-500 | µAmes Bacterial Mutation Test Kit | - | 1 sample kit |
Phenotype Test Kit
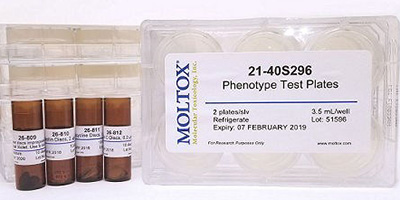
Specially designed to confirm all the phenotype characteristics listed by the OECD 471 guideline in any of the E. coli and Salmonella strains used in the Ames test
Kit contents:
- 6-well phenotype confirmation test plates
- 4 types of antibiotic discs for phenotype characterization
| Catalog no. | Description | Storage | Size |
|---|---|---|---|
| 31-600 | Phenotype Test Kit | - | to test 6 strains |
1 Maron D., Ames B. Revised methods for the Salmonella mutagenicity test. Mutation Research 113(3-4):173-215, 1983.
2 Green M.H., Muriel W.J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutation Research 38(1):3-32, 1976.
3 OECD Guideline 471 for the Testing of Chemicals, Section 4. Test No. 471: Bacterial Reverse Mutation Test
4 Guideline: ICH M7 Assessment and Control of DNA reactive (mutagenic) Impurities in Pharmaceuticals to limit potential carcinogenic risk
Matsushima T., Sawamura M., Hara K. and Sugimura T. A Safe Substitute for Polychlorinated Biphenyls as an Inducer of Metabolic Activation Systems. In: "In vitro Metabolic Activation in Mutagenesis Testing", Eds F.J. de Serres et al. Elsevier, North Holland, pp. 85-88, 1976.



